We are learning a mechanism that is the exact opposite of Fischer Esterification .That means instead of going from a Carboxylic Acid to an Ester, we are hydrolyzing an Ester to a Carboxylic Acid . 1 concept General Reaction 1m 4 Comments Mark as completed Was this helpful? 3 2 concept General Mechanism 2m 6 Comments Mark as completed
The reaction shown below illustrates the acid-catalyzed hydrolysis of an acetal into a hemiacetal. Draw the complete arrow pushing mechanism that accounts for products shown above. | Homework.Study.com
First, we have to identify the functional group of the reactant. The reactant is an ester, which has the general formula RCOOR’. In this case, the ester is methyl benzoate, which has the formula C6H5COOCH3. Second, we have to remember that acid-catalyzed hydrolysis of an ester produces a carboxylic acid and an alcohol.

Source Image: quizlet.com
Download Image
Science Chemistry Draw the organic product for the following acid-catalyzed hydrolysis reaction. H2SO4, H20 Draw the organic product for the following acid-catalyzed hydrolysis reaction. H2SO4, H20 BUY Chemistry for Today: General, Organic, and Biochemistry 9th Edition ISBN: 9781305960060
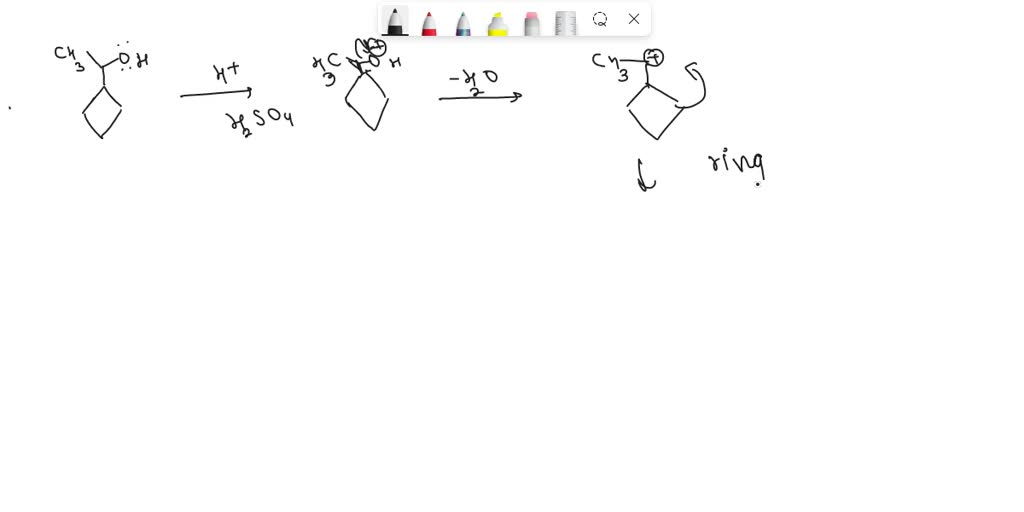
Source Image: numerade.com
Download Image
Answered: 6) Which is the organic product for the… | bartleby Mar 7, 2023In acid-catalyzed hydrolysis, a strong acid such as sulfuric acid or hydrochloric acid is used to provide the necessary protons to break apart the chemical bonds in the molecule. The addition of water molecules to the broken bonds then leads to the formation of smaller molecules.
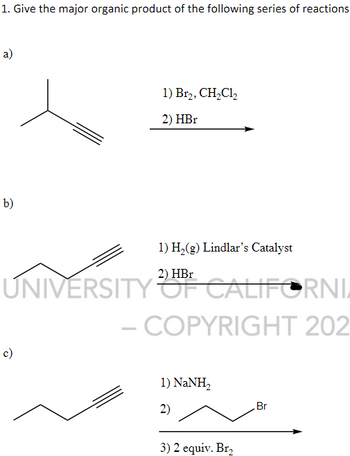
Source Image: bartleby.com
Download Image
Draw The Organic Product For The Following Acid-Catalyzed Hydrolysis Reaction.
Mar 7, 2023In acid-catalyzed hydrolysis, a strong acid such as sulfuric acid or hydrochloric acid is used to provide the necessary protons to break apart the chemical bonds in the molecule. The addition of water molecules to the broken bonds then leads to the formation of smaller molecules. Oct 17, 2022Organic Chemistry I 6: Alkenes
Answered: Give the major organic product of the… | bartleby
Voiceover: In the video on Fischer esterification we saw that if we took a carboxylic and alcohol, in an acid-catalyzed reaction, we produced an ester, and we also produced water. Our goal in that video was to make more of our ester, so we shifted the equilibrium to the right, to make more of our product. In this video, we’re talking about the SOLVED: (10 points) Draw the mechanism for the following acid-catalyzed acetal hydrolysis. If resonance structures exist for any intermediates, make sure to show them: H2O OCH3 CH3OH H2O OH (12 points) a)

Source Image: numerade.com
Download Image
Solved) – Draw the organic products you would expect to isolate from the… – (1 Answer) | Transtutors Voiceover: In the video on Fischer esterification we saw that if we took a carboxylic and alcohol, in an acid-catalyzed reaction, we produced an ester, and we also produced water. Our goal in that video was to make more of our ester, so we shifted the equilibrium to the right, to make more of our product. In this video, we’re talking about the

Source Image: transtutors.com
Download Image
The reaction shown below illustrates the acid-catalyzed hydrolysis of an acetal into a hemiacetal. Draw the complete arrow pushing mechanism that accounts for products shown above. | Homework.Study.com We are learning a mechanism that is the exact opposite of Fischer Esterification .That means instead of going from a Carboxylic Acid to an Ester, we are hydrolyzing an Ester to a Carboxylic Acid . 1 concept General Reaction 1m 4 Comments Mark as completed Was this helpful? 3 2 concept General Mechanism 2m 6 Comments Mark as completed

Source Image: homework.study.com
Download Image
Answered: 6) Which is the organic product for the… | bartleby Science Chemistry Draw the organic product for the following acid-catalyzed hydrolysis reaction. H2SO4, H20 Draw the organic product for the following acid-catalyzed hydrolysis reaction. H2SO4, H20 BUY Chemistry for Today: General, Organic, and Biochemistry 9th Edition ISBN: 9781305960060
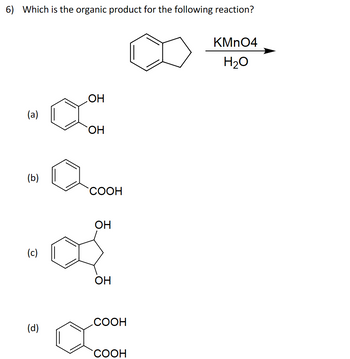
Source Image: bartleby.com
Download Image
Mechanism of Acid-Catalyzed Hydrolysis of An Ester with a Tertiary Alkyl Group | Organic chemistry, Chemistry lessons, Chemistry 5: Organic Chemical Reactions

Source Image: pinterest.com
Download Image
Hydrolysis of an acetal in aqueous acid gives an aldehyde or a ketone and two molecules of alcohol or one molecule of a diol. Draw the structural formulas for the products of Mar 7, 2023In acid-catalyzed hydrolysis, a strong acid such as sulfuric acid or hydrochloric acid is used to provide the necessary protons to break apart the chemical bonds in the molecule. The addition of water molecules to the broken bonds then leads to the formation of smaller molecules.

Source Image: homework.study.com
Download Image
Answered: Draw the organic products formed in… | bartleby Oct 17, 2022Organic Chemistry I 6: Alkenes
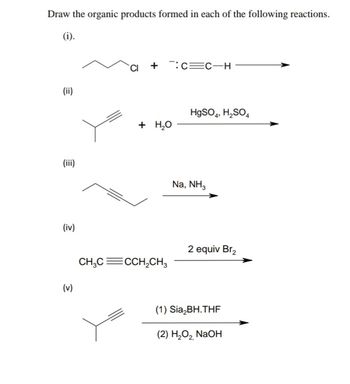
Source Image: bartleby.com
Download Image
Solved) – Draw the organic products you would expect to isolate from the… – (1 Answer) | Transtutors
Answered: Draw the organic products formed in… | bartleby First, we have to identify the functional group of the reactant. The reactant is an ester, which has the general formula RCOOR’. In this case, the ester is methyl benzoate, which has the formula C6H5COOCH3. Second, we have to remember that acid-catalyzed hydrolysis of an ester produces a carboxylic acid and an alcohol.
Answered: 6) Which is the organic product for the… | bartleby Hydrolysis of an acetal in aqueous acid gives an aldehyde or a ketone and two molecules of alcohol or one molecule of a diol. Draw the structural formulas for the products of 5: Organic Chemical Reactions