Trimethylammonium Ion. Structural Formula C 3 H 10 N trimethylammonium ion Molecular Model.
Lesson 4.4: Energy Levels, Electrons, and Covalent Bonding – American Chemical Society
The electron configurations of ions are those of the neutral atoms plus or minus a number of electrons equal to the charge on the ion. Groups 15 to 17 Write the electronic structure for the neutral atom. Then add electrons to get a total of eight. E.g., for “Cl”^-, we get “Cl”: 1s^2 color (white) (l)2s^2 2p^6color (white) (l) 3s^2 3p^5 but “Cl

Source Image: pinterest.com
Download Image
This page titled 3.2: Ions and the Octet Rule is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by Lisa Sharpe Elles. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Electron configurations can be used to show how many electrons are needed to complete an octet and form an ion.

Source Image: pinterest.com
Download Image
How Many Valence Electron Does Silicon (Si) Have? | Electrons, Silicone, Atomic number ‘How many valence electrons are in the trimethylammonium ion (CH;),NH Donienov Mlleeelal’ AI Recommended Answer: There are six valence electrons in the trimethylammonium ion. Best Match Video Recommendation: Solved by verified expert QM Qbs Main. We don’t have your requested question, but here is a suggested video that might help.
Source Image: valenceelectrons.com
Download Image
How Many Valence Electrons Are In The Trimethylammonium Ion
‘How many valence electrons are in the trimethylammonium ion (CH;),NH Donienov Mlleeelal’ AI Recommended Answer: There are six valence electrons in the trimethylammonium ion. Best Match Video Recommendation: Solved by verified expert QM Qbs Main. We don’t have your requested question, but here is a suggested video that might help. A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = 6 valence electrons Group 17 = 7 valence electrons Group 18 = 8 valence electrons (except helium)
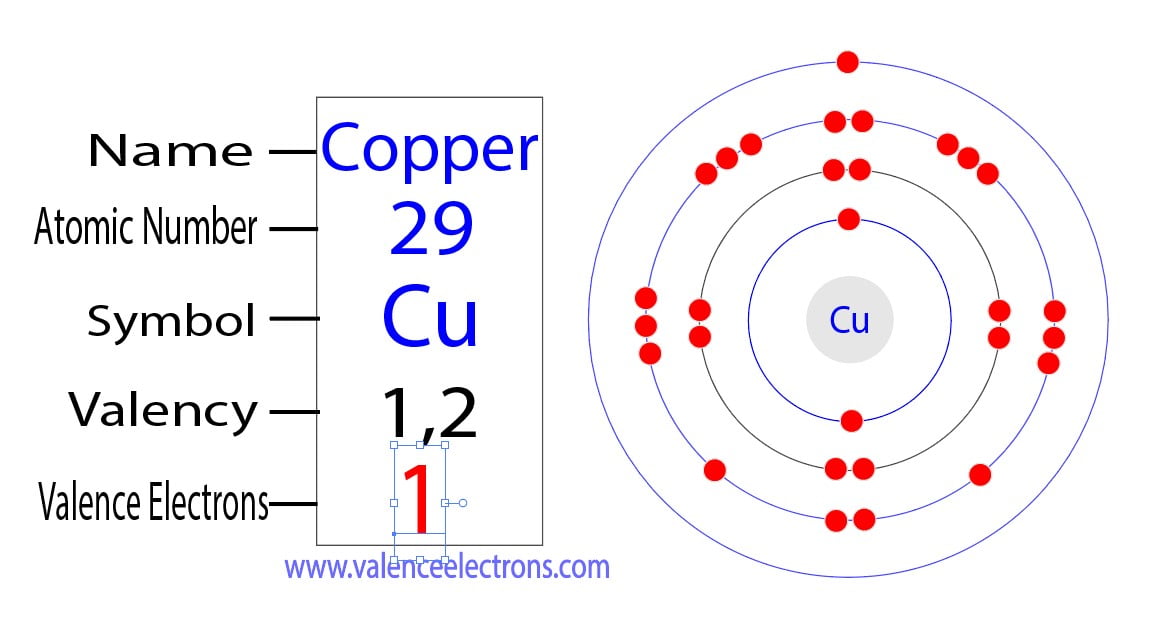
How to Find the Valence Electrons for Copper (Cu)?
Let us determine the Lewis structures of SiH 4, CHO 2 −, NO +, and OF 2 as examples in following this procedure: Determine the total number of valence (outer shell) electrons in the molecule or ion. For a molecule, we add the number of valence electrons on each atom in the molecule: SiH 4 Si: 4 valence electrons/atom × 1 atom = 4 + H: 1 2022: ☢️ Valence Electrons in Technetium (Tc) [& Facts, Color, Discovery …

Source Image: materials.gelsonluz.com
Download Image
Aleks Counting valence electrons in an atomic ion – YouTube Let us determine the Lewis structures of SiH 4, CHO 2 −, NO +, and OF 2 as examples in following this procedure: Determine the total number of valence (outer shell) electrons in the molecule or ion. For a molecule, we add the number of valence electrons on each atom in the molecule: SiH 4 Si: 4 valence electrons/atom × 1 atom = 4 + H: 1

Source Image: m.youtube.com
Download Image
Lesson 4.4: Energy Levels, Electrons, and Covalent Bonding – American Chemical Society Trimethylammonium Ion. Structural Formula C 3 H 10 N trimethylammonium ion Molecular Model.

Source Image: acs.org
Download Image
How Many Valence Electron Does Silicon (Si) Have? | Electrons, Silicone, Atomic number This page titled 3.2: Ions and the Octet Rule is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by Lisa Sharpe Elles. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Electron configurations can be used to show how many electrons are needed to complete an octet and form an ion.

Source Image: pinterest.com
Download Image
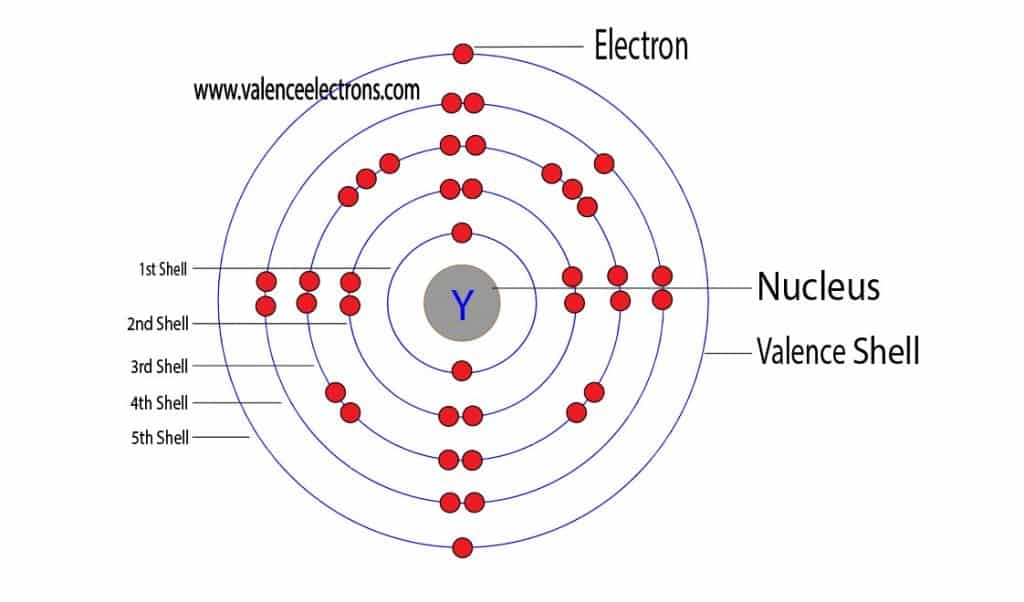
How to Find the Valence Electrons for Yttrium (Y)? Figure 8.4.1 8.4. 1: (a) The radius of an atom is defined as one-half the distance between the nuclei in a molecule consisting of two identical atoms joined by a covalent bond. The atomic radius for the halogens increases down the group as n increases. (b) Covalent radii of the elements are shown to scale.
Source Image: valenceelectrons.com
Download Image
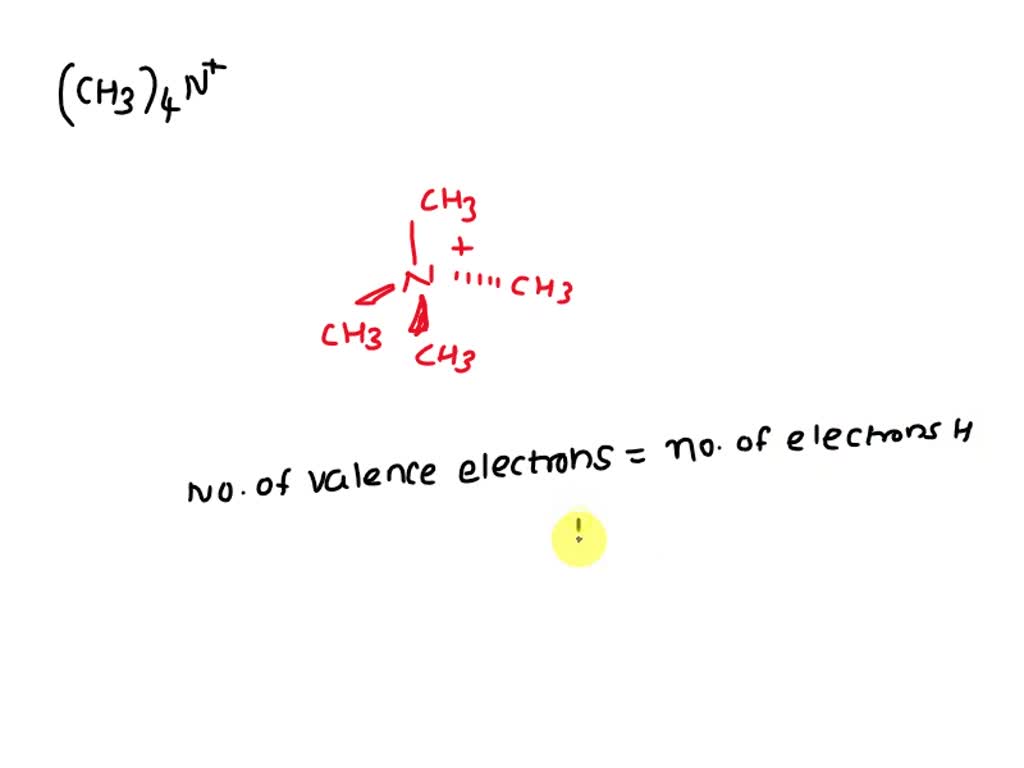
SOLVED: How many valence electrons are there in the tetramethylammonium ion, (CH3)4N+? a. 32 b. 18 c. 8 d. 33 e. 24 ‘How many valence electrons are in the trimethylammonium ion (CH;),NH Donienov Mlleeelal’ AI Recommended Answer: There are six valence electrons in the trimethylammonium ion. Best Match Video Recommendation: Solved by verified expert QM Qbs Main. We don’t have your requested question, but here is a suggested video that might help.
Source Image: numerade.com
Download Image
Titanium (Ti) Electron configuration, Orbital diagram, and Valence electrons | Electron configuration, Aufbau principle, Electrons A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = 6 valence electrons Group 17 = 7 valence electrons Group 18 = 8 valence electrons (except helium)

Source Image: pinterest.com
Download Image
Aleks Counting valence electrons in an atomic ion – YouTube
Titanium (Ti) Electron configuration, Orbital diagram, and Valence electrons | Electron configuration, Aufbau principle, Electrons The electron configurations of ions are those of the neutral atoms plus or minus a number of electrons equal to the charge on the ion. Groups 15 to 17 Write the electronic structure for the neutral atom. Then add electrons to get a total of eight. E.g., for “Cl”^-, we get “Cl”: 1s^2 color (white) (l)2s^2 2p^6color (white) (l) 3s^2 3p^5 but “Cl
How Many Valence Electron Does Silicon (Si) Have? | Electrons, Silicone, Atomic number SOLVED: How many valence electrons are there in the tetramethylammonium ion, (CH3)4N+? a. 32 b. 18 c. 8 d. 33 e. 24 Figure 8.4.1 8.4. 1: (a) The radius of an atom is defined as one-half the distance between the nuclei in a molecule consisting of two identical atoms joined by a covalent bond. The atomic radius for the halogens increases down the group as n increases. (b) Covalent radii of the elements are shown to scale.